OT-AFM Combi-System
Combined system with optical tweezers and AFM
The Bruker JPK OT-AFM Combi-System combines:
- AFM: Surface force measurement and high resolution imaging
- Optical tweezers: 3D positioning, detection and manipulation
The set-up is highly modular; using the unique ConnectorStage you can combine any NanoWizard or CellHesion AFM with the NanoTracker optical tweezers on a research-grade inverted optical microscope.
- Imaging, positioning, and manipulation experiments from single molecules to living cells
- Measure forces in 2D and 3D, from 500fN to 10nN, on the same sample
- Combine any NanoTracker and NanoWizard /CellHesion 200 system
- Compatible with optical microscopy techniques eg TIRF and confocal
- Wide range of modes and accessories
Contact us for more information and quotes:
+44 (0)1223 422 269 or info@blue-scientific.com

Single Molecule Applications with up to 14 Degrees of Freedom
OT-AFM significantly extends the range of single-molecule applications. A variety of handles, interaction and detection sites are available for your studies – as in the example below:
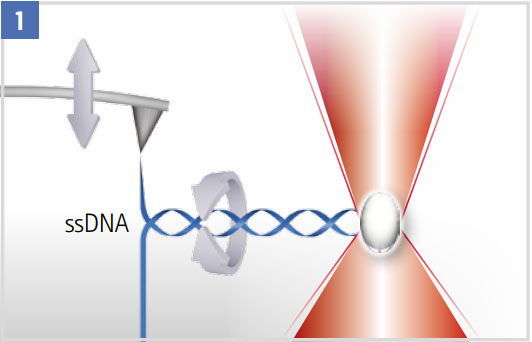
Unzipping a DNA Hairpin
The optical trap can be used to suppress (high laser power) or quantify rotation (low laser power).
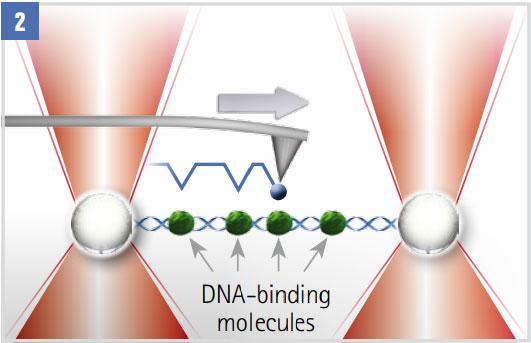
Scanning a Decorated DNA Molecule
The molecule with DNA binding proteins (green) spans two optically trapped beads. A functionalised AFM tip (blue) scans the molecule. Interactions between the DNA-attached proteins and the tip are detected in the AFM and OT signals.
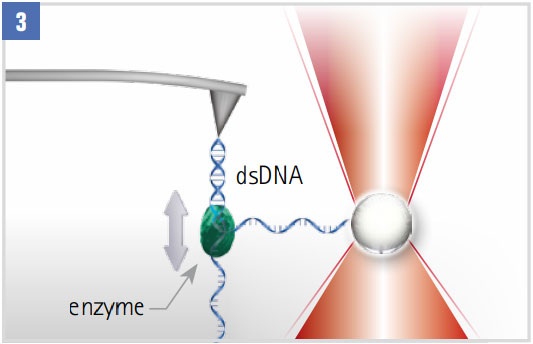
Monitoring DNA-enzyme Dynamics (eg polymerase, helicase)
With one strand attached to an optically trapped particle, the step-wise motion is tracked. Closed-loop force clamping maintains a constant force on the single strand.
Live Cell Applications
With the JPK OT-AFM you can combine AFM, optical tweezers and fluorescence microscopy to study:
- Cellular response
- Cell-cell or cell matrix interactions
- Immune response
- Infection
- Bacterial/virus/nanoparticle uptake processes
Cells are activated with functionalised beads along with parallel AFM measurement. Signaling molecules on the surface of a micro-particle are brought in contact with the cell at defined positions and time points.
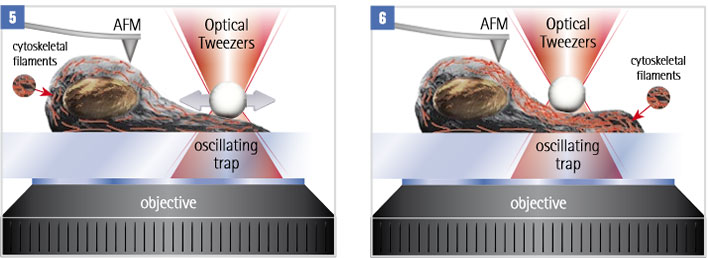
A mechano-sensitive cell is stimulated by a periodic force, exerted by an optically trapped particle. Internal rearrangements of the cytoskeleton change the mechanical properties of the cell. These properties can be analysed with AFM methods like force mapping or JPK’s Quantitative Imaging Advanced (QI-Advanced) mode.
AFM can be used in parallel to monitor changes in the cell structure. For example monitoring mechanical properties throughout the process. Or use molecular recognition force spectroscopy to study the distribution and mechanical behaviour of membrane proteins.
Trigger Immune Signaling to influence Cell Adhesion
A useful technique is using functionalised particles or modified microorganisms to trigger cellular responses. AFM can then be used to measure changes in cellular structure, dynamics and mechanical properties. Using alternative methods, it can be difficult to deliver objects to specific areas of the cell.
Optical tweezers are perfect for manipulating the sample and triggering cellular responses at specific times and areas.
In this example, OT-AFM is used to quantify the influence of signaling between dendritic cells (DCs) and regulatory T-cells (Treg) on the adhesion of conventional T-cells (Tconv) to the same DC.
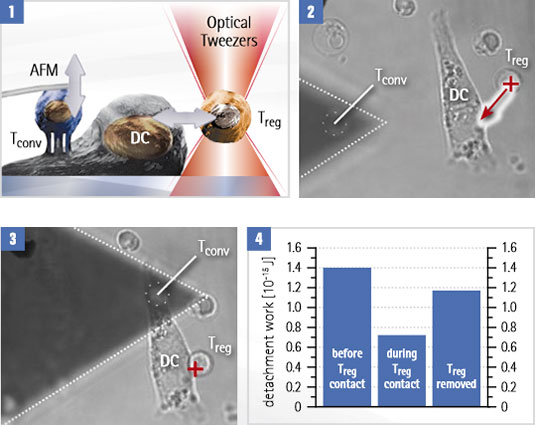
Adhesion experiment with dendritic cells (DC) and conventional T-cells (Tconv)
- The Tconv is attached to a tipless cantilever, then moved closer to the surface-bound DC. The cantilever is pulled up and the adhesion forces are measured. A regulatory T-cell (Treg) is attached to and removed from the DC with optical tweezers, to test its influence on the binding strength.
- The optical trap (red cross) moves the Treg while adhesion is measured with Tconv attached to a cantilever.
- Detachment work is measured for each. Treg attachment reduces DC-T conv interactions. After the Treg is removed, the adhesion level is almost restored.
Courtesy of Yan Shi, University of Calgary/Tsinghua University, Beijing. Experiment designed by Yan Shi et al. (publication in print).

