Using Optical Tweezers to Manipulate and Measure Forces
An overview of how optical tweezers can be used to manipulate live cells and measure forces, including:
- Intracelullar measurement
- Adhesion
- Particle-cell interaction
- Membrane properties
- Cell mechanics
- Indentation
Further details about the applications mentioned in this article are available in this pdf from JPK.
Blue Scientific is the official distributor for Bruker JPK instruments in Norway, Sweden, Denmark, Finland and Iceland. For more information or quotes, please get in touch.
NanoTracker 2 optical tweezers
Contact us on +44 (0)1223 422 269 or info@blue-scientific.com
Follow @blue_scientificOptical Tweezers
Optical tweezers (aka optical traps) use highly focused lasers to trap and manipulate objects at the nano- and microscale. They’re used in many biological and biophysical studies.
You can hold and position a variety of objects, from particles on the scale of tens of nanometers up to whole cells. The forces acting on the trapped objects can be measured extremely precisely, down to single pico-newtons and below. This opens up a broad range of possibilities in cellular research.
The technique involves transfering momentum from light entering a refractive particle for stabilisation in a trapping potential. A full explanation of how optical tweezers work is available in this technical note from JPK.

Bruker JPK NanoTracker 2
- Optical tweezers platform
- 3D force measurements with femto-Newton sensitivity and sub-nm precision
- Simultaneous fluorescence imaging
- Suitable for all applications in this article – and more
Manipulate Cells
Suspended cells usually have a higher refractive index than their surrounding medium, so they can be trapped easily with optical tweezers. You can then hold and position the suspended cells in 3D.
The laser is non-invasive, causing only moderate absorption and heating for biological samples (near-infrared wavelength 1064nm) .
With the JPK NanoTracker 2, you can employ several traps to manipulate multiple particles or cells simultaneously.
Example: Cell Sorting and Adhesion
In the example below, yeast cells are gathered in a glass micro-capillary using optical tweezers. Using the NanoTracker’s software, the cells can be sorted and deposited on patterned growth substrates with an easy “drag and drop” technique.
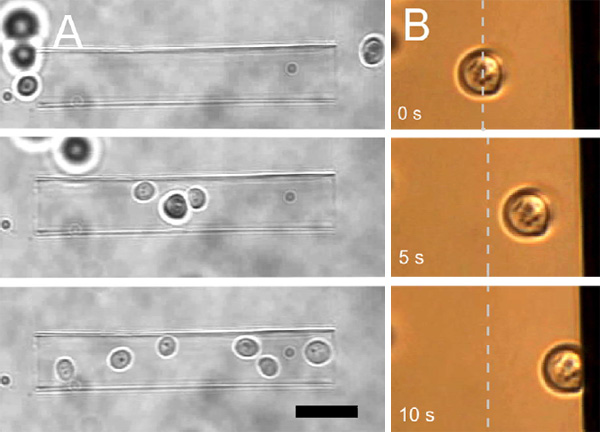
On the right in the example above, a yeast cell is touched against a vertical surface. The cell can be held in contact with the substrate to observe its behavior. The contact time and force is varied to measure cell adhesion. This technique can be used with pre-treated surfaces, for example to assess the bio-compatibility of implants. Other bacteria or microorganisms can be used to study anti-fouling treatments in medical and technical applications.
Microfluidics
Further possibilities open up by using an integrated microfluidic system (JPK LaminarFlowCell aka LFC). Cells can be exposed to streams containing drugs or nutrients, to observe their responses.
More about the Laminar Flow Cell
Measuring Forces
For simple cells such as yeast and some unicellular organisms with well-defined shapes and optical properties, the trap can be calibrated to determine stiffness and measure forces on the organism. The trapped cell is exposed to a known force and its displacement is measured, eg by pushing through a viscous medium. The drag force depends on the object’s size and shape, velocity and the viscosity. This enables you to measure external forces acting on the cell and binding forces in adhesion processes. For example, this technique has been used to measure the adhesion forces of sickle-shaped Malaria parasites during surface binding.
Measuring Forces on Cells with Irregular Shapes
For optically inhomogeneous cells with irregular shapes, measuring absolute force values can be challenging. This can be overcome by using defined, spherical particles such as micrometer-size polystyrene or silica beads. These can be attached to cells as handles and force probes.
Beads are available in a wide variety of sizes, materials, surface chemistries and fluorescent labeling. These can be used to perform complex experiments, without needing to be an expert in surface modification.
Particle-Cell Interaction
The manipulation of cells and beads can be used to trigger biochemical reactions and quantify the forces acting during cellular processes. Beads carrying signaling molecules, receptors or other functional groups can be used to study the interaction of molecules with cellular signaling pathways.
For example, you could trigger cellular processes simply by bringing functionalised beads into contact with a cell. This method has been used to activate individual immune cells, by bringing antigens into contact with different areas of their surface.
Intracellular Force Measurement
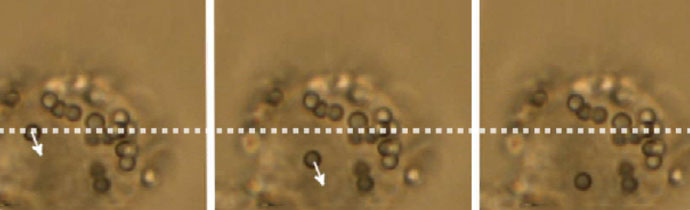
Force measurements and particle manipulation are not restricted to the surface of cells. After a cell has engulfed a particle via endocytosis or cellular uptake processes, it can still be trapped and moved.
In the example below, a polystyrene bead is being moved inside a macrophage cell. Intracellular force measurements involve more complex calibration methods, taking into account the refractive index and viscosity of the cytosol, as well as the possible inhomogeneity of the cell interior.
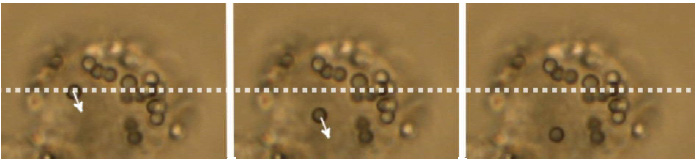
There are many possibilities for measurements inside living cells:
- Trap cellular organelles and other substructures eg measure the forces of kinesin motor proteins transporting lipid droplets in micro-tubules.
- Rheological measurements eg determine the viscoelastic properties in various areas inside the cell.
- Micro-scale granules can be oscillated at different amplitudes and frequencies. The displacement provides information about the loss and storage module of the intracellular material (corresponding to viscous and elastic contributions).
Membrane Properties
The outer cell membrane is highly complex structure that directly interacts with other cellular components such as the cytoskeleton. The mechanical properties of the cell membrane (tension, viscosity) are important in many processes involved in cell signaling, motility or growth. Abnormal changes are related to malfunctions and diseases. It’s therefore extremely useful to be able to characterise the cell membrane in varied environmental conditions and at different stages of cell proliferation and differentiation.
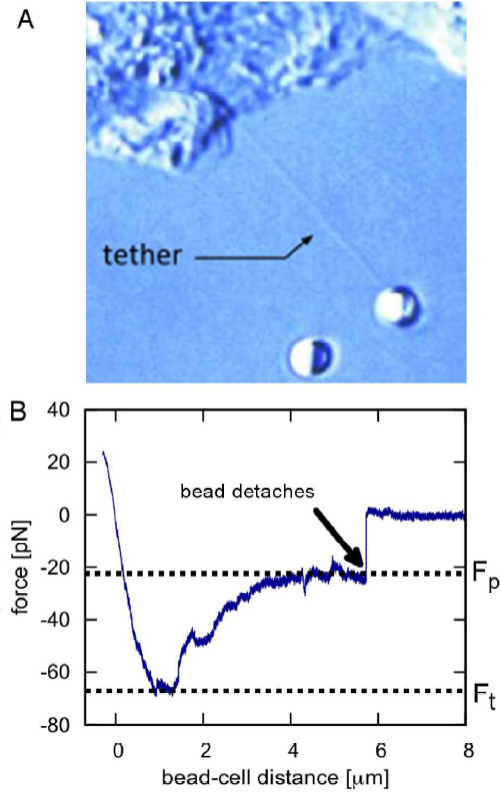
Information about the local mechanical properties of the membrane can be acquired from tether-pulling experiments. Beads coated with immunoglobulin G (IgG) antibodies are trapped and attached to the cell membrane. By applying force to the bead, micrometer long membrane tubes (tethers) are pulled out and the forces on the attached particle are recorded. This provides insights into mechanisms including cell motility,
vesicle trafficking and intercellular signaling.
Cell Mechanics
The mechanical properties of cells are closely related to their function. Single cell rheology is therefore extremely useful for identifying cell types and detecting (mal-)functions. Cells in tissue and those adhered to rigid substrates can be measured using bulk rheology and AFM, but these techniques are not suitable for freely floating cells, such as those in the blood or lymph. It can be tricky to measure the viscoelasticity of cells suspended in a native fluid environment, under physiological conditions.
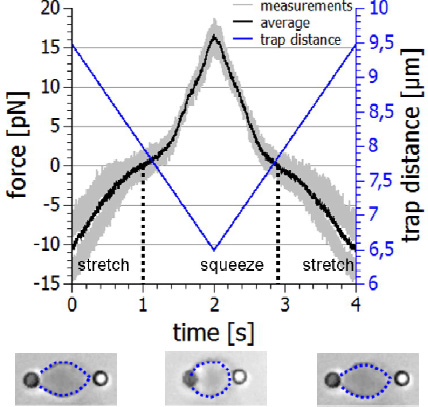
With the NanoTracker 2 you can study the mechanics of suspended cells using functionalised beads that bind to their surface or the underlying cytoskeleton. Two beads on opposite sides of the cell surface can be used as handles. The optical tweezers can then stretch or squeeze the cell with adjustable frequencies and amplitudes, and record the viscoelastic response.
The example below shows an erythrocyte mechanics experiment. The cell is held with two beads in a dual optical trap. By moving one of the trapswith acousto-optical deflectors (AODs), the cell is repeatedly stretched and squeezed and the forces acting on the beads are recorded. By plotting the force-distance relationship, you can determine important information about the mechanical properties of the cell and its physiological state. As well as live blood cells, erythrocyte ghosts consisting mainly of the cell’s membrane can also be studied to evaluate the effects of drug treatments on the membrane.
Indentation
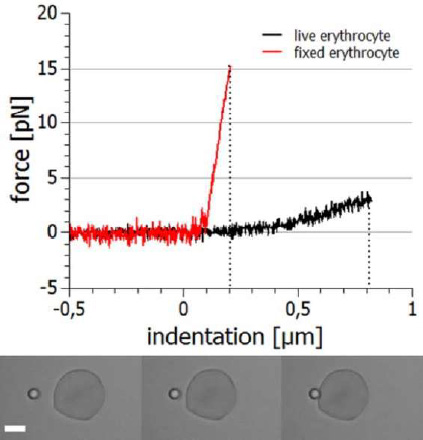
Other aspects of cellular viscoelasticity can be assessed by fixing the cell in space (eg aspirated by a micro-pipette or adhered to a surface) and indenting with a microparticle. The indentation depth will be in the range of tens to hundreds of nanometres, so high stability and accurate positioning and measurement is essential.
The example below shows the approach and retraction segments of this type of experiment. Indenting a live cell requires much lower forces (approx. 3 pN for 800 nm) than a fixed cell (approx. 15 pN for 200 nm). The fixation agent increases the stiffness.
These types of experiments give you useful insights into the link between biological function and mechanical properties. This can be used to identify potential targets for new drugs that regulate cell function by influencing cell mechanics and their related dynamics.
Further details about the applications mentioned in this article are available in this pdf from JPK.
More Information
NanoTracker 2 optical tweezers can be used for a wide variety of cell-related experiments from cell sorting and single molecule interactions to the whole cell mechanics. The effects of drugs and functional molecules on cellular behaviour and mechanics can also be studied, and there are further possibilities with microfluidics.
Blue Scientific is the official distributor of Bruker JPK systems in the Nordic region. For more information about how optical tweezers could be used in your research, and for quotes for the NanoTracker 2, please get in touch: