Analysing Plant Cell Walls with AFM – Paper and Pulp
AFM (Atomic Force Microscopy) is a powerful tool for imaging plant cell walls, to study their structure and the orientation of microfibrils. This type of analysis provides insights that are useful in the paper and pulp industry.
Researchers at the Department of Biology and Center for Lignocellulose Structure and Formation at Penn State University have published a paper on this subject entitled “Spatial organisation of cellulose microfibrils and matrix polysaccharides in primary plant cell walls as imaged by multichannel atomic force microscopy”.
Blue Scientific is the official distributor of Bruker AFM instruments in the Nordic region (Sweden, Finland, Denmark, Iceland and Norway). If you have any questions about AFM or if you’d like a quote, please get in touch:
Bruker Dimension Icon AFM
Read the scientific paper
Contact us on +44 (0)1223 422 269 or info@blue-scientific.com
Studying the Structure of Plant Cell Walls
AFM, together with complementary electron microscopy was used to characterise the nanoscale and mesoscale structure of the outer (periclinal) cell wall of onion scale epidermis. This is a model system for studying wall structure in relation to cell wall mechanics.
The epidermal wall consists of around 100 lamellae, each approx. 40 nm thick. These contain 3.5-nm wide cellulose microfibrils oriented in a common direction within a lamella, varying by ~30 to 90° between adjacent lamellae. The wall has a crossed polylamellate structure, rather than being helicoidal.
Montages of high resolution AFM images were taken of the newly deposited wall surface. These showed that single microfibrils merge into and out of short regions of microfibril bundles, to create a reticulated network. Microfibril direction within a lamella did not change gradually or abruptly across the whole face of the cell; they were found to be consistent across the outer wall.
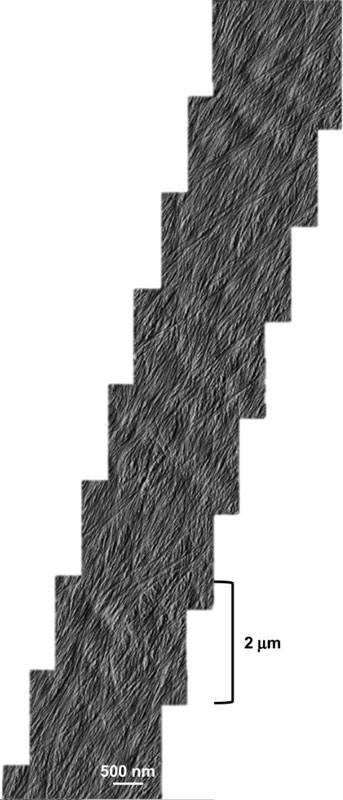
Montage of high resolution AFM images showing extensive microfibril bundling. Microfibrils merge into and out of bundles, forming a reticulated network.
A layer of pectin on the surface of the wall obscured the underlying cellulose microfibrils when imaged by FESEM. However with AFM they were visible. AFM was able to image through the soft matrix of the hydrated walls.
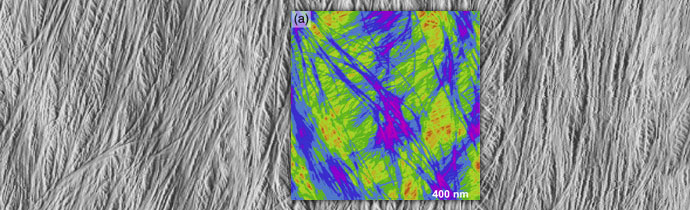
AFM-based nanomechanical mapping revealed significant heterogeneity in cell wall stiffness and adhesiveness at the nanometre scale. Colour coding and combining maps hihglighted the spatial distribution of soft and rigid matrix polymers, in the context of the stiffer microfibrils.
The results provide multi-scale structural information about the primary cell wall at close to native state, without chemical extraction or dehydration. This has implications for microfibril motions in different lamellae during uniaxial and biaxial extensions.
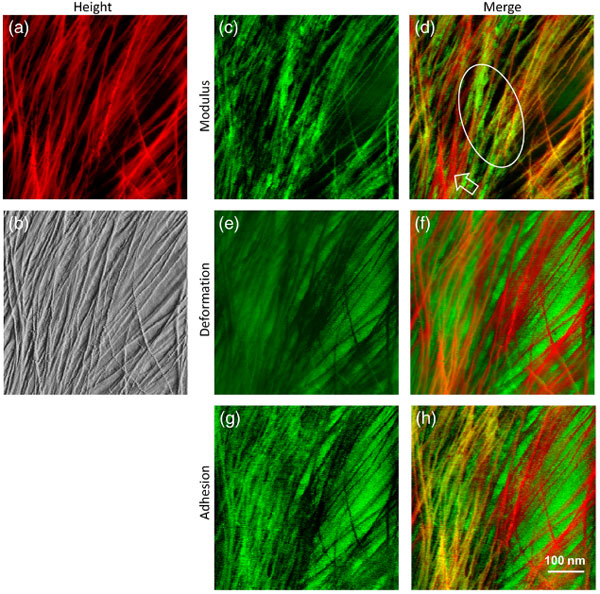
Multi-channel AFM images showing the heterogeneity of cell wall polymer distribution: (a) Height image of onion epidermal walls. (b) PeakForce error image, (c) modulus map, (e) deformation map, and (g) adhesion map captured simultaneously of the same area. (d) Merged from (a) and (c). Hollow arrow heads point to the flexible microfibrils with low moduli. Ellipse indicates non-fibrillar rigid regions. (f) Merged from (a) and (e). (h) Merged from (a) and (g).
Bruker Dimension Icon AFM
The atomic force microscope used in this study was the Bruker Dimension Icon, a large sample AFM suitable for a wide range of samples:
- Widest range of AFM modes and applications
- Quantitative nanoscale material property mapping
- Simultaneous nanoscale imaging and nanomechanical analysis
- Lower noise floor and higher accuracy than any other large sample AFM
AFM-Raman
AFM can also be combined with Raman spectroscopy in a single instrument:
- Co-localised data about a sample’s topography and chemical composition
- Insights into the mechanisms behind specific material properties
- Advanced AFM modes including electrical characterisation and quantitative nanomechanical mapping
Read the Paper
The scientific paper by Penn State University is available to read in The Plant Journal, with full discussion of results and images. It’s currently available to read online for free.
If you have any questions about AFM or the Dimension Icon and how it could be used in your area of research, please get in touch: